Describe the Shape of a Water Molecule
You will probably hear the term water vapor which means water in a gas state. Describe the most recent version of the fluid mosaic model of membrane structure.
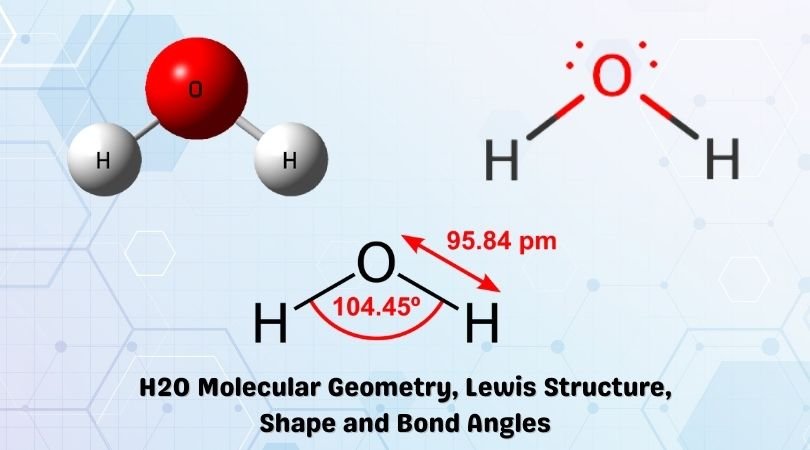
H2o Molecular Geometry Lewis Structure Shape And Bond Angles
One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.

. The terms dominant and recessive describe the inheritance patterns of certain traits. The permanent dipole of each water molecule polarizes all adjacent water molecules. Dipole-induced dipole interactions are always attractive and can contribute as much as 05 kcalmole 21 kjoulemole to stabilization of molecular associations.
It is important that rain and water are not absorbed through the leaves as this would disrupt the flow of nutrients which rely on the passage of water from root to leafIf the water were allowed to travel by osmosis through the cell membrane and into the leaf it would change the osmotic pressure in the leaves and. The molecules polarity and especially its ability to form hydrogen bonds makes ammonia highly miscible with water. This geometry is a direct result of the repulsion experienced by the four groups of bonding electrons.
Good examples of these types of liquids include water H 2 O and mercury Hg. The lone pair makes ammonia a base a proton acceptor. Water is a Polar Covalent Molecule.
The two atoms in a diatomic molecule are connected in a straight line. It is linear in shape and has no polarity. The shape of this molecule is a result of the electrons in the four bonds positioning themselves so as to minimize the repulsive effects.
Vapor and gas mean the same thing. Identify the types and numbers of molecules that provide the free energy necessary for the reduction of the PGA molecules. But what do they really mean.
What molecule does the PGA molecule turn into during this phase of the Calvin cycle. Describe the general structure of a phospholipid molecule and what makes it suitable as a major component of cell membranes. Browse the archive of articles on Nature Chemistry.
It is common for multiple strands of mRNA to be translated simultaneously by multiple ribosomes. Collect your molecules and view them in 3D. The word vapor is used to describe gases that are usually liquids at room temperature.
Many plants have hydrophobic coatings on their leaves. Once the water molecule is added to the polypeptide the polypeptide is released from the ribosome. G3P contains more H atoms that PGA c.
Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour. The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two poles a positive charge on the hydrogen pole side and a negative charge on the oxygen pole side. Explain the behavior of a great number of phospholipid molecules in water.
This can easily be observed in a. A wide variety of covalent organic cages and two- and three-dimensional covalent organic frameworks have been obtained through dynamic covalent. A polypeptide chain must fold on itself to create its final shape as a protein.
The dipole of a water molecule induces change in the dipoles of all nearby water molecule. Starting from atoms see how many molecules you can build. Light energy causes the removal of an electron from a molecule of P680 that is part of Photosystem II.
Water is the chemical substance with chemical formula H 2 O. A fourth weak force also has a central role in determining the shape of a proteinAs described in Chapter 2 hydrophobic molecules including the nonpolar side chains of particular amino acids tend to be forced together in an aqueous environment in order to minimize their disruptive effect on the hydrogen-bonded network of water molecules see p. The molecular geometry which is what describes the shape of a diatomic molecule is representative of linear geometry.
They get the vapor title when they are in a gaseous phase. Describe specifically how the structures of the two molecules in part a are different. However if you have to describe the ion you can use the phrase the like a polar molecule because I3- is soluble in water.
58 and Panel 2-2 pp. On the other hand the ammonia molecule NH 3 also has four electron pairs associated with the nitrogen atom and thus has a tetrahedral electron-pair geometry. This greatly increases the rate of protein production.
In concluding remarks to sum up this entire article I3- is a polyatomic ion that has 22 valence electrons 3 lone pairs 2 bond pairs and sp3d hybridization. This shape gives the molecule a dipole moment and makes it polar. RNAs chemical structure gives it the flexibility to take on a variety of shapes and functions.
The shape we see is the only possible shape for a central carbon atom with four bonds. The energy transfer is similar to the chemiosmotic electron transport occurring in the mitochondria. One of these regions however is a lone pair which is not included in the molecular structure and this lone pair influences the shape of the molecule Figure PageIndex5.
The P680 requires an electron which is taken from a water molecule breaking the water into H ions and O-2 ions.

Molecular Shapes Chemistry For Non Majors
Comments
Post a Comment